
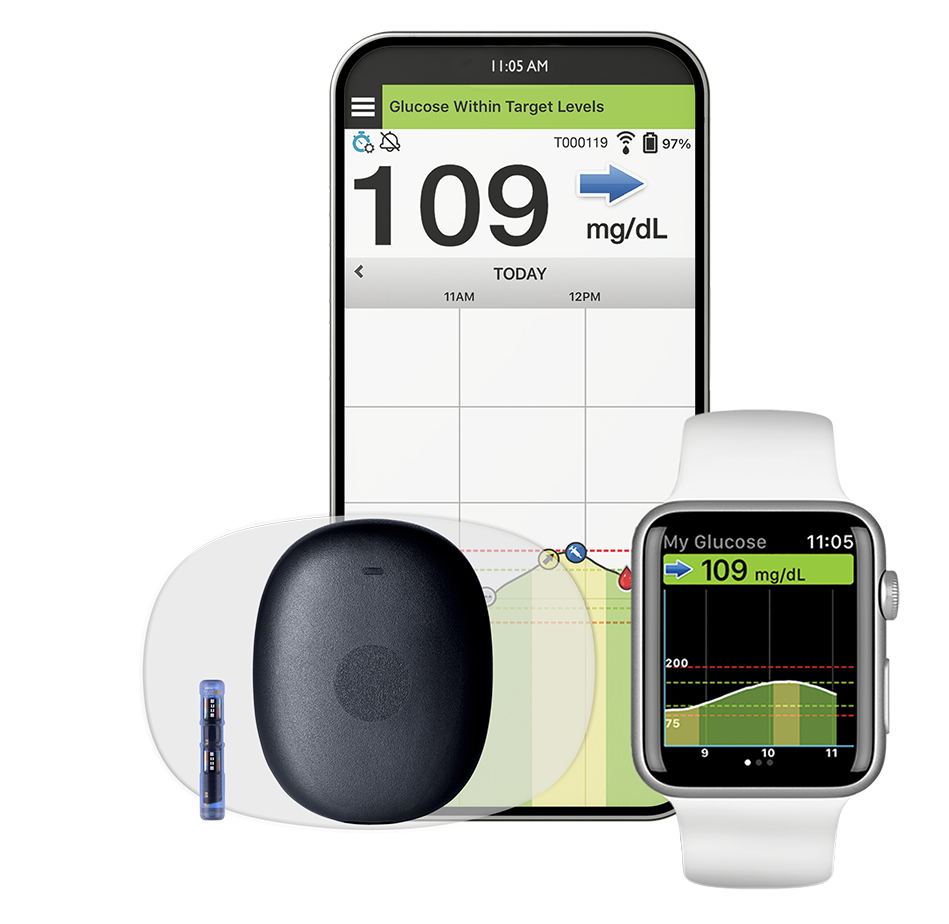
GERMANTOWN, MD, & ST. LOUIS – Senseonics Holdings, Inc. (NYSE American: SENS) (“Senseonics” or the “Company”), a medical technology company focused on the development and manufacturing of long-term, implantable continuous glucose monitoring (CGM) systems for people with diabetes, and Mercy, a leading healthcare system, today announced that the first commercial patient received the next-generation Eversense® 365 CGM system. This milestone, part of the company’s previously announced collaboration with Mercy, marks the world’s first commercial use of Eversense 365, the only CGM to provide one-year of accurate monitoring with minimal interruptions.
This patient and those who follow, along with their doctors, will be able to monitor glucose levels and trends for a full year, with no requirement for additional sensor replacements and only one calibration per week. Eversense 365 offers patients a truly differentiated CGM experience, enabling confident decisions, long-term peace of mind and enhanced quality of life with just one CGM. It also helps people to overcome common frustrations and interruptions experienced with traditional, short-term CGMs that are indicated to last just 10-14 days. Senseonics and Mercy expect this significant breakthrough in diabetes technology and management to improve glycemic control for patients, reduce distress associated with managing diabetes and lower overall healthcare costs for providers and payers.
"Without the technology, patients deal with constant finger pricks to test their glucose or have sensors that need to be replaced every few days,” said Dr. Jeff Ciaramita, Mercy’s president of specialty service lines. “This is a pivotal moment for people with diabetes because this groundbreaking technology allows patients a more convenient and reliable way to manage their condition for an entire year with a single sensor. As Mercy strives to make care easy for our patients, what better way than to be the first in the world to offer Eversense 365.”
Eversense 365 was approved by the U.S. Food and Drug Administration last month. The commercial launch, which has now begun, is being managed by Senseonics global commercial partner, Ascensia Diabetes Care, a subsidiary of PHC Holdings Corporation (TSE 6523). Mercy serves 3 million patients annually and expects that approximately 30,000 of Mercy’s patients could benefit from a CGM system.
"We are thrilled to see the successful first commercial insertion of the Eversense 365 CGM system at Mercy," said Francine Kaufman, M.D. Senseonics’ chief medical officer. "Eversense 365 addresses many of the challenges that patients experience with traditional, short-term CGMs and we are excited to see the real-world benefit it can have as it is rolled out to patients across the U.S. We expect that health systems across the country will recognize the benefits that Mercy has embraced and can adopt Eversense 365 towards enhancing the quality of care while reducing the cost of care. Our goal is to make this life-changing technology accessible to as many patients as possible through collaborations with forward-thinking health care leaders such as Mercy."
“This long-term monitoring solution empowers patients with real-time data and enables our clinicians to deliver proactive, personalized care, ultimately leading to better health and quality of life for those we serve," Dr. Ciaramita said. “By adopting Eversense 365, we're enhancing our commitment to value-based care ‒driving to improve patient outcomes while reducing overall health care costs.”
To learn more about Eversense 365 and for additional information about when the system will be available, go to www.eversensecgm.com. Physicians, nurse practitioners and physician assistants who are interested in offering the Eversense CGM System can sign up at https://provider.eversensecgm.com/contact-us/. Alternatively, contact 1-844-SENSE4U (1-844-736-7348) to learn more about the first and only long-term implantable CGM system.

About Senseonics
Senseonics Holdings, Inc. ("Senseonics") is a medical technology company focused on the development and manufacturing of glucose monitoring products designed to transform lives in the global diabetes community with differentiated, long-term implantable glucose management technology. Senseonics' CGM systems Eversense® 365 and Eversense® E3 include a small sensor inserted completely under the skin that communicates with a smart transmitter worn over the sensor. The glucose data are automatically sent every 5 minutes to a mobile app on the user's smartphone.
About Eversense
The Eversense® Continuous Glucose Monitoring (CGM) Systems are indicated for continually measuring glucose levels for up to 365 days for Eversense® 365 and 180 days for Eversense® E3 in persons with diabetes age 18 and older. The systems are indicated for use to replace fingerstick blood glucose (BG) measurements for diabetes treatment decisions. Fingerstick BG measurements are still required for calibration primarily one time per week after day 14 for Eversense® 365 and one time per day after day 21 for Eversense® E3, and when symptoms do not match CGM information or when taking medications of the tetracycline class. The sensor insertion and removal procedures are performed by a health care provider. The Eversense CGM Systems are prescription devices; patients should talk to their health care provider to learn more. For important safety information, see https://www.eversensediabetes.com/safety-info/.
About Ascensia Diabetes Care
Ascensia Diabetes Care is a global company focused entirely on helping people with diabetes. Our mission is to empower those living with diabetes through innovative solutions that simplify and improve their lives.
We are home to the world-renowned CONTOUR® portfolio of blood glucose monitoring systems and the exclusive global distribution partner for the Eversense® Continuous Glucose Monitoring Systems from Senseonics. These products combine advanced technology with user-friendly functionality to help people with diabetes manage their condition and make a positive difference to their lives. As a trusted partner in the diabetes community, we collaborate closely with healthcare professionals and other partners to ensure our products meet the highest standards of accuracy, precision and reliability, and that we conduct our business compliantly and with integrity.
Ascensia is a member of PHC Group and was established in 2016 through the acquisition of Bayer Diabetes Care by PHC Holdings Corporation. Ascensia products are sold in more than 100 countries. Ascensia has around 1,400 employees and operations in 29 countries.
For further information, please visit the Ascensia Diabetes Care website at: http://www.ascensia.com
About PHC Holdings Corporation
PHC Holdings Corporation (TSE 6523) is a global healthcare company with a mission of contributing to the health of society through healthcare solutions that have a positive impact and improve the lives of people. Its subsidiaries (referred to collectively as PHC Group) include PHC Corporation, Ascensia Diabetes Care Holdings AG, Epredia Holdings Ltd., LSI Medience Corporation, Mediford Corporation, and Wemex. Together, these companies develop, manufacture, sell and service solutions across diabetes management, healthcare solutions, life sciences and diagnostics. PHC Group’s consolidated net sales in FY2023 were JPY 353.9 billion with global distribution of products and services in more than 125 countries.
©2024 Ascensia Diabetes Care Holdings AG. All right reserved. Ascensia, the Ascensia Diabetes Care logo and Contour are trademarks and/or registered trademarks of Ascensia Diabetes Care Holdings AG.

